Dr. Md. Rashidul Haque
MBBS, FCPS (Psychiatry)
Member, American Psychiatric Association
Member, European Psychiatric Association.
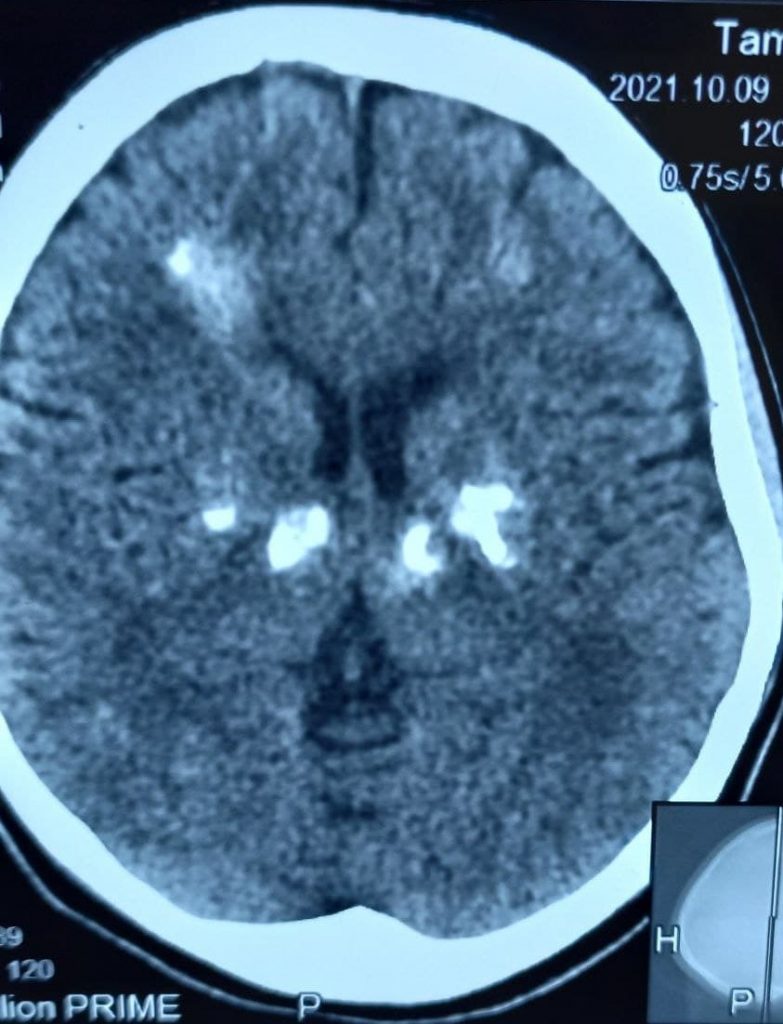
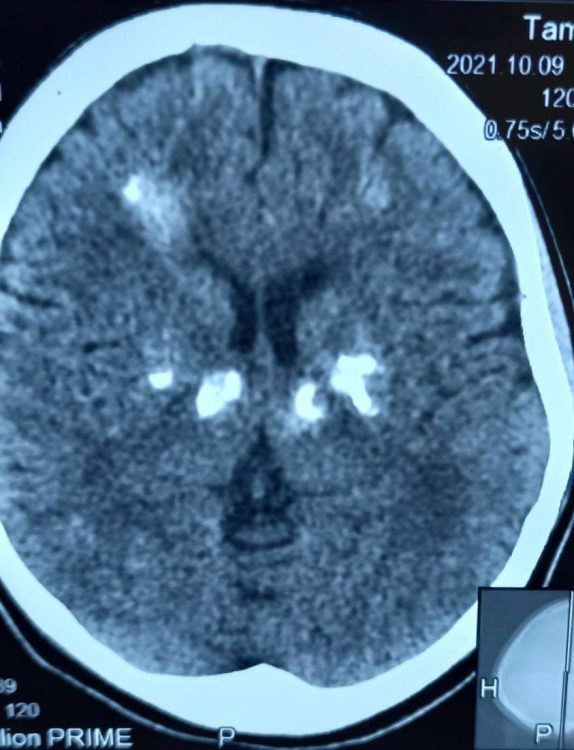
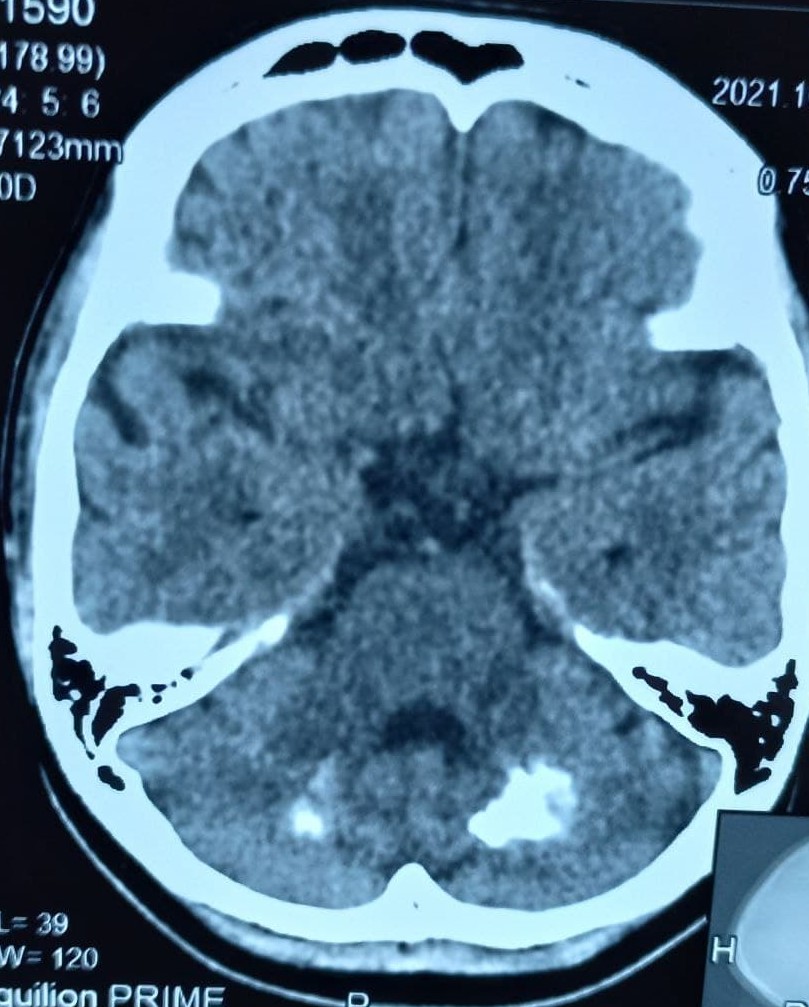
Fahr’s disease or Fahr’s syndrome is a rare, neurological disorder characterized by abnormal calcified deposits in
basal ganglia and cerebral cortex.
It is also known as familial idiopathic basal ganglia calcification or primary familial brain calcification.
Calcified deposits are made up of calcium carbonate and calcium phosphate, and are commonly located in the Basal Ganglia, Thalamus, Hippocampus, Cerebral cortex, Cerebellar Subcortical white
matter, and Dentate Nucleus.
It was first described by German neurologist Karl Theodor Fahr in 1930.
It is a rare inherited or sporadic neurological disorder with a prevalence of <1/1,000,000. Fahr’s syndrome commonly affects young to middle-aged adults typically individuals in the 3rd and 4th decades of their lives.
Clinical features:
1 Neurological: Loss of consciousness, tetany, seizures, gait disorder, spasticity, speech impairment, dementia, myoclonus, coma, paroxysmal choreoathetosis, dystonic choreoathetosis, papilledema of intracranial, hypertension, pleocytosis of CSF.
2 Movement disorder: Clumsiness, fatigability, unsteady gait, involuntary movements, and muscle cramping
3 Neuropsychiatric features: Psychosis, depression, apoplexia, deterioration of intelligence, inability to make decisions.
Diagnostic criteria:
Diagnostic criteria of Fahr’s syndrome has been modified and derived from Moskowitz et al. 1971, Ellie et al. 1989, Manyam 2005 and it can be stated as follows:
a. Bilateral calcification of the basal ganglia visualized on neuroimaging. Other brain regions may also be
observed.
b. Progressive neurologic dysfunction, which generally includes a movement disorder and/or neuropsychiatric manifestations. Age of onset is
typically in the fourth or fifth decade, although this dysfunction may also present in childhood.*
c. Absence of biochemical abnormalities and somatic features suggestive of mitochondrial or metabolic disease or other systemic disorder.
d. Absence of an infectious, toxic, or traumatic cause.
e. Family history consistent with autosomal dominant inheritance.

Pathogenesis:
The calcium deposits usually form within the vessel wall and in the perivascular space and finally extend to the neuron. The pathogenesis of this syndrome is believed to be due to defective iron transport and free radical production, resulting in tissue damage. This leads to the initiation of brain calcification. This calcification compresses the vessel lumen, initiating a cycle of impaired blood flow and injury to neurons. Most calcifications occur bilaterally and symmetrically while a few occur unilaterally.
Etiology:
Fahr’s disease is most commonly transmitted as an Autosomal Dominant trait, but it may also be passed on as an autosomal recessive trait or it may occur sporadically. A Locus at 14q (IBGC1) has been suggested to
be involved commonly. A second locus has been identified on chromosome 8 and a third on chromosome 2. A loss of function mutation in the gene encoding type III sodium-dependent phosphate transporter 2 (SLC20A2) located on chromosome 8 has also been reported as the molecular level to form the genetic basis for the pathophysiology of this disease.
Differential diagnoses:
Hyperparathyroidism, Pseudo-hypoparathyroidism, Tuberous sclerosis, Toxoplasmosis, Rubella, CNV, Herpes infection during intr-uterine life and Syphilis, and inflammatory conditions like Systemic lupus erythematosus.
Investigations:
Routine lab results are within normal limits in a patient with Fahr disease. Any abnormality should prompt further investigations to rule out secondary causes of brain calcifications.
- Routine hemogram and complete metabolic panel
- Blood and urine heavy metal levels
- Serum calcium, phosphorus, magnesium, alkaline phosphatase (ALP), calcitonin, vitamin D, parathyroid hormone (PTH)
- Cerebrospinal fluid analysis for bacteria, virus, and parasites
- Ellsworth Howard test (following 200 U PTH injection, 10 to 20 fold increase in urinary cAMP levels)
Molecular Genetic Testing: This is done in an index case that meets the diagnostic criteria to establish the diagnosis of Fahr disease. There are three approaches to genomic testing:
- Serial gene testing: Sequentially testing for genes one at a time, based on the frequency of causation
SLC20A2>PDGFB>PDGFRB>XPR1
- Multigene panel: One-time analysis of all the four implicated pathogenic variants (SLC20A2, PDGFB, PDGFRB, XPR1) and other genes to rule out secondary syndromes
- Comprehensive genomic testing
- CT – scan of brain used to assess the location and severity of cerebral calcification.

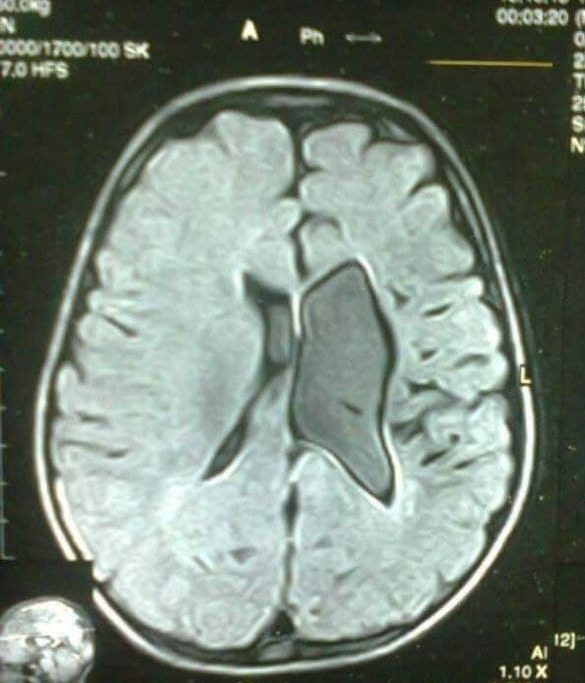
- MRI of brain – generally less useful than CT, may be used to locate calcification in the brain, but appearance varies depending on stage of disease and the amount of calcification.
- X-ray skull – may be used to locate symmetrical calcium clusters lateral to midline.
Management:
The management of Fahr’s patients aims at symptomatic support.
Pharmacological treatment should be used to improve the condition of the patient. Antiepileptics are used for seizures and atypical antipsychotic drugs for psychosis.
Seizures and movement disorders that are associated with parathyroid dysfunction can be resolved with the correction of phosphate and calcium levels by prescribing them alpha hydroxyvitamin D3 and corticosteroids.
Oxybutynin is used for urinary incontinence.
Prognosis:
The prognosis for any individual with Fahr’s Syndrome is variable and hard to predict. There is no reliable correlation between age, the extent of calcium deposits in the brain, and neurological deficit.